Details of the Drug
General Information of Drug (ID: DMV74ZH)
| Drug Name |
Pizotyline
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Pizotifen; PIZOTYLINE; 15574-96-6; Sandomigran; Litec; Sandomygran; Pizotifenum; Pizotifeno; Polomigran; Pizotifene; BC-105; BC 105; Pizotyline [USAN]; Pizotifan; Pizotifene [INN-French]; Pizotifenum [INN-Latin]; Pizotifeno [INN-Spanish]; UNII-0BY8440V3N; EINECS 239-632-9; BRN 0753534; C19H21NS; CHEBI:50212; FIADGNVRKBPQEU-UHFFFAOYSA-N; Pizotifen (INN); Pizotifen [INN]; 0BY8440V3N; Pizotyline (USAN); 4-(9,10-dihydro-4H-benzo[4,5]cyclohepta[1,2-b]thiophen-4-ylidene)-1-methylpiperidine
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
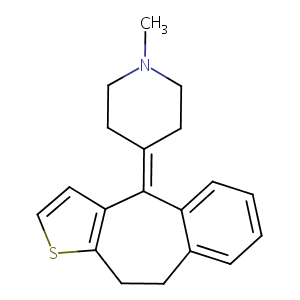 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 295.4 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.8 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
